Moderna has their african (beta?) variant shot in trials. It’ll probably come in several months before Pfizer’s, but still towards the end of the year. My wild guess is their multivalent (original + .351 targeted) shot will be favored, and be effective against african variant and be a little better than original for P.1 (Brazillian) variant not forgetting the original.
In any case, if someone gets a shot now, it’ll likely be safe to get a shot of an updated booster when they come out later.
so 2ANZ, then 1 PFE and we’ll wait for the variants booster till EOY
Depends on your risk tolerance or risk vs reward and outlook on symptomatic infection vs protection against hospitalization.
Look at the design of the clinical studies where biontech/moderna test their boosters. The conditions/interval between shots should be informative. No guarantee, but informative.
I saw Merkel got 1 AZN, then PFE. I haven’t seen any 2 AZN then PFE etc studies.
I guess the UK might do that soon given how delta is spreading there.
In any case my parents are doing 2 AZN then 1st PFE today, will follow studies etc to see when to do 2nd .Reminds me a lot of flu variants and the guess/probability game we play everyyear
pharmacist would’nt let them get PFE since they had AZN already. Will have to try another…
I guess they have been watching CNBC https://www.cnbc.com/2021/07/20/dr-vin-gupta-encourages-jj-vaccine-recipients-to-get-a-pfizer-or-moderna-booster.html?recirc=taboolainternal
Worked at another location
The vaccine is also now eligible for off-label prescriptions—or use beyond the approved populations. That could include booster doses, according to the FDA.
Get your 3rd doses today, totally legitimately, as long as you can persuade your doctor to write you a script.
Good article on the booster considerations
Including why “waning” vaccine immunity could well be a statistical artifact from giving it to old people first (whose immune systems aren’t as good and are more at risk of covid anyway) and from using an unvaccinated control group who may increasingly contain people who already had an asymptomatic case and hence likely won’t get sick again (making the vaccine comparison look less good).
But the main thing is we don’t have a full trial showing this helps but the politicians and drug companies are happy to push boosters for their own agendas.
In short, diminished vaccine effectiveness does not make the case for boosters. A reduction in severe outcomes makes the case for boosters, but we have no such data to date.
Boosters are an important medical question. Their approval must have a favorable safety and efficacy profile. Only randomized trials measuring severe disease can show that.
Put another way - immunity is waning, but we aren’t sure if it’s because of time, or because it was given to old people first. I’m sure the WHO will disagree, but assuming these countries have plenty of vaccine, it sounds perfectly reasonable to target boosters to these who got it first.
FDA saying there’s not enough evidence to support boosters, and warning Biden to back off. He’s been promoting them at 8mo post vaccination, then 6mo, then 5mo (seriously).
Several senior FDA officials resigned over the White House pressure for boosters, in case you missed that. Follow the politics, not the science.
Yes, I have extreme confidence that the research, testing, and approval of the vaccine(s) was not politically guided/cajoled/influenced-in-any-way. It is also a certainty that the vaccine(s) is/are, as the CDC/FDA/NYT/ABC/NBC/CBS/PBS/TiananmenTimes verified years ago, perfectly safe and effective.
Over 3 billion people have gotten at least one dose already, the problem cases are probably less than one in ten million. So yes, perfectly safe. Probably safer than some other vaccines we regularly use.
Given that > 90% of the people who end up in hospitals, ICUs, and 6 feet under are unvaccinated, the vaccines are obviously effective.
I’m not scared of the vaccine but you’re overselling the lack of risks with 1 in 10M.
- Strokes from CVT is often deadly at 1 in 1M
- low platelet and clotting issues, serious, at 1 in 100k
- myocarditis, not often as severe but still not good at 1 in 50k or so
Not all of these apply to all risk groups equally or all vaccines, but bad cases are out there.
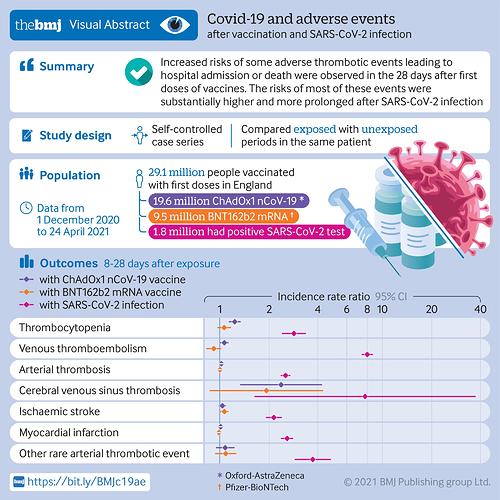
That said, this article had a nice graph of the risks of the vaccine for these rare, sometimes serious side effects, and the corresponding risk of the same health issues if you got covid. Covid was nearly always worse by a factor of several, so if you think covid is going to be endemic and everyone gets it, better to get it with a vaccine instead of not. See the bottom for relative risk of PFE vaccine, AZN vaccine, and covid.
“Probably”. That’s says it all.
And while the side effects may be “rare”, the CDC also says it is “rare” for a person recovered from an infection to not retain natural immunity. Yet they still push how essential it is for the recovered to be vaccinated. Either “rare” means still too much potential risk, or it means we can ignore the potential risk; someone has to get their story straight before people are going to buy in either way.
I don’t agree with your definition of perfectly safe. Using your descriptors above, more like “probably safe”.
ETA: I did not see @glitch99’s response before babbling the above.
Got my 2nd PFE in Jan. Debating whether to wait since I have no underlying issues (maybe prediabetic) What’s the verdict w/ mix and match?
A UK trial for 2nd dose mix and match showed different was better. No study results on 3rd dose yet that I know of, probably ongoing presently.
Unless you’d like to live like a hermit, and maybe even if you do, I’d say it’s inevitable. Just a question of when.